Contributors to this page: ICRISAT, Patancheru, India (RP Thakur, AG Girish, VP Rao).
Scientific name
Verticillium dahliae Kleb. and Verticillium albo–atrum Reinke & Berthie
Other scientific names
Verticillium albo-atrum var. chlamydosporale, Verticillium albo-atrum var. dahliae, Verticillium albo-atrum var. medium, Verticillium dahliae f. chlamydosporale, Verticillium dahliae f. medium, Verticillium ovatum, Verticillium tracheiphilum.
Importance to CGIAR centers
High
Significance
Verticilliumdahliae affects many important crops including peanut and causes losses of economic significance in many countries.
Symptoms
Early symptoms usually appear at the flowering stage and include marginal chlorosis of the leaves, loss of leaf turgidity and leaf curling. Leaf symptoms are generally yellowing and leaflet necrosis, followed by wilting and defoliation. The roots of the infected plants have brown discoloration of the vascular tissues. Occasionally plants die, and the roots of the dead plants are severely rotted (Subrahmanyam et al. 1992).
Hosts
Verticilliumdahliae has a very wide host range among economically important crops such as Gossypium (cotton), Solanum tuberosum (potato), Solanum melongena (aubergine), Capsicum annuum (bell pepper), Olea europaea subsp. europaea (olive), Brassica napus var. napus (rape), Fragaria ananassa (strawberry), Humulus lupulus (hop), Lycopersicon esculentum (tomato), Medicago sativa (lucerne), Mentha (mints),Arachis hypogaea (groundnut), Armoracia rusticana (horseradish), Brassica oleracea var. gemmifera (Brussels sprouts), Pistacia vera (pistachio), Prunus (stone fruit) and Vitis vinifera (grapevine).
Geographic distribution
Verticilliumdahliae is worldwide in distribution, including Asia, Africa, Europe, USA and Australia.
Biology and transmission
Verticilliumdahliae is moderately to fast-growing fungus with little to moderate aerial mycelium and a regular margin, turning black from the centre after a week due to production of microsclerotia. Conidiophores are verticillate and conidiogenous cells subtended in whorls (2-3 per node), and are erect and hyaline. Conidia are ellipsoidal, hyaline, mostly one-celled and produced at the tips of narrow, pointed sterigmata. Conidia are 2.5-6 ´ 1.5-3.0 µm in size. Conidia are produced in succession to form moist spore balls at the tips of conidiogenous cells, giving characteristic appearance to conidiophore in culture. Microsclerotia are of irregular shape and size (50-200 ´ 15-100 µm), dark brown to black and globose. The fungus survives in soil as microsclerotia which germinate in response to root exudates. The hyphae or germinating conidia penetrate the cortex of young roots and the fungus grows into the stele. In the xylem vessels the pathogen spreads by mycelial growth, and also by the production of conidia which get into transpiration stream. Microsclerotia are formed in senescing diseased tissues. The pathogen is disseminated throughout the field soil by farm equipment, wind and water movement and by infected seed.
Detection/indexing methods used in CGIAR at ICRISAT
- Pre export field inspection and blotter test are used.
Treatment/control
- Not available.
Procedures followed in case of positive test at ICRISAT
- Incineration of the infected plants and rejection of the infected seed samples.
EPPO protocols
EPPO A2 list: No. 85
Detection. Use of DNA hybridization probes (Robb et al. 1990) and ELISA test for V. albo-atrum are in use in France for testing certified pelargonium (OEPP/EPPO 1992).
Phytosanitary risk. EPPO has listed hop-infecting strains of V. albo-atrum and V. dahliae as A2 quarantine pests (OEPP/EPPO 1982), but no other regional plant protection organization has done so. Regulatory control may remain appropriate, but may take on the character of a certification scheme for planting material.
Phytosanitary Measures. EPPO recommends (OEPP/EPPO 1990) that hop planting material should come from a field where verticillium wilt has not occurred in the last 5 years and that consignments and their mother plants should have been found free from the disease in the last growing season. Such measures are as relevant in a national certification scheme as for international phytosanitary certification.
References and further reading
OEPP/EPPO. 1982. Data sheets on quarantine organisms No. 85, Hop-infecting strains of Verticillium albo-atrum and V. dahliae. Bulletin, OEPP/EPPO Bulletin12 (1).
OEPP/EPPO. 1990. Specific quarantine requirements. EPPO Technical DocumentsNo. 1008.
OEPP/EPPO. 1992. Certification schemes No. 3. Pathogen-tested material of pelargonium. OEPP/EPPO Bulletin22: 285-296.
Robb J, Hu X., Schmidt J, Nazar R. 1990. DNA hybridization probes for the identification and quantification of V. dahliae and V. albo-atrum. In: Abstracts of the 5th International Verticillium Symposium, Leningrad, USSR, p. 97.
Subrahmanyam P, Wongkaew S, Reddy DVR, Demski JW, McDonald D, Sharma SB, Smith DH. 1992. Field diagnosis of groundnut diseases. Information bulletin no. 36, Patancheru, AP, 502 324, India: International Crops Research Institute for the Semi Arid Tropics. 84pp.
 Verticillium wilt (Verticillium dahliae) of groundnut: marginal chlorosis, leaf curling, tiger striping and yellowing of leaves(photo:ICRISAT) |
Scientific name
Colletotrichum dematium (Pers.) Grove.
Other scientific names
Colletotrichum bakeri, Colletotrichum brassicae, Colletotrichum lysimachiae, Colletotrichum pucciniophilum, Colletotrichum sanguisorbae, Colletotrichum volutella, Dinemasporium dianthi, Ellisiellina volutella, Sphaeria dematium, Vermicularia bakeri, Vermicularia dematium, Vermicularia dianthi, Vermicularia echinata, Vermicularia lagunensis, Vermicularia lysimachiae, Vermicularia volutella.
Importance to CGIAR centers
Low
Significance
Anthracnose in peanut is of minor importance.
Symptoms
Symptoms appear as wedge-shaped lesions on the leaflet tips. Lesions may also develop on the leaflet margins leading to marginal blight. The periphery of the advancing margins of the lesion is surrounded by the yellow zone. The necrotic tissue becomes dark brown and tends to fragment along the leaflet margins. The disease may also extend to stipules and stems. Fruiting bodies (acervuli) are visible through a hand lens, and are abundant on infected leaf tissue (Subrahmanyam et al. 1992).
Hosts
Allium cepa (onion), Allium porrum (leek), Allium sativum (garlic), Arachis hypogaea (groundnut), Beta vulgaris (beetroot), Helianthus annuus (sunflower), Piper betle (betel pepper), Vicia faba (broad bean), Voandzeia subterranea (bambara groundnut), Abelmoschus esculentus (okra), Allium (onions, garlic, leek, etc.), Cicer arietinum (chickpea), Capsicum annuum (bell pepper), Crotalaria juncea (sunn hemp), Glycine max (soyabean), Lablab purpureus (hyachinth bean), Lycopersicon esculentum (tomato), Vigna radiata (bean, mung), Vigna mungo (black gram), Spinacia oleracea (spinach).
Geographic distribution
Colletotrichum dematium is worldwide in distribution, especially India, Niger, Nigeria, Sudan, Senegal, Taiwan, Tanzania, Thailand, Uganda and USA.
Biology and transmission
Mycelium of C.dematium is hyaline, it produces circular, errumpt, dark brown to black acervuli. These acervuli are scattered on the infected pods or aggregated or in groups. Acervuli exude spores in pale to smoke-gray masses. Numerous thick, black, erect setae are interspersed within the acervuli. Conidia are hyaline, 1-celled and 2.5-4.0 ´ 15-32 mm in size. They are fusoid and bluntly tapered at both ends (Ahmed and Ravinder Reddy 1993).
Detection/indexing methods used in CGIAR at ICRISAT
- Pre export field inspection and blotter test are used.
Treatment/control
- Not available.
Procedures followed in case of positive test at ICRISAT
- Rejection of seed samples in case of positive test.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Subrahmanyam P, Wongkaew S, Reddy DVR, Demski JW, McDonald D, Sharma SB, Smith DH. 1992. Field diagnosis of groundnut diseases. Information bulletin no. 36, Patancheru, AP, 502 324, India: International Crops Research Institute for the Semi Arid Tropics. 84pp.
 Anthracnose (Colletotrichum dematium) of groundnut: (A)wedge-shaped lesions on the leaflet; (B)fungal growth on seed; (C)acervuli with setae on seed and (D)conidia (photos:ICRISAT) |
Scientific names
Rhizoctonia bataticola (Tassi) E.J. Butler, Macrophomina phaseolina (Tassi) Goid.
Other scientific names
Botryodiplodia phaseoli, Dothiorella cajani, Dothiorella phaseoli, Dothiorella philippinensis, Fusicoccum cajani, Macrophoma cajani, Macrophoma corchori, Macrophoma phaseoli, Macrophoma phaseolina, Macrophoma sesami, Macrophomina philippinensis, Rhizoctonia lamellifera, Sclerotium bataticola, Tiarosporella phaseoli, Tiarosporella phaseolina.
Importance
High
Significance
Charcoal rot is economically important across a broad range of crops throughout the world, particularly in regions that experience hot, dry conditions during the growing period. Yield losses in groundnut of 100, 94 and 63% have been reported when disease appeared at the pre-emergence, pre-pod and pod-filling stages, respectively (Sharma and Bhowmik 1986).
Symptoms
Water-soaked lesions appear on the hypocotyl near the soil surface. The lesions enlarge, become dull brown, girdle the hypocotyle, and kill the plants. Lesions on the roots appear water-soaked at first, but infected tissues eventually have a dull, light-brown appearance. Later, affected areas become covered with sclerotia. Roots become rotten and blackened with shredding of the taproot. The dead tissues rot and turn black, as sclerotia of the fungus develop profusely. Infected pegs and pods also rot and become covered with sclerotia.
Hosts
Many crop plants including Allium cepa (onion), Allium sativum (garlic), Arachis hypogaea (groundnut), Beta vulgaris var. saccharifera (sugarbeet), Brassica oleracea var. botrytis (cauliflower), Cajanus cajan (pigeon pea), Carthamus tinctorius (safflower), Cicer arietinum (chickpea), Cyamopsis tetragonoloba (clusterbean), Coriandrum sativum (coriander), Capsicum annuum (bell pepper), Cucumis melo (melon), Cucumis sativus (cucumber), Curcuma longa (turmeric), Crocus sativus (saffron), Glycine max (soyabean), Helianthus annuus (sunflower), Nicotiana tabacum (tobacco), Oryza sativa (rice), Pennisetum glaucum (pearl millet), Vigna radiata (bean, mung), Vigna mungo (black gram), Phaseolus vulgaris (common bean), Sorghum bicolor (common sorghum), Vigna unguiculata (cowpea) and Zea mays (maize).
Geographic distribution
Rhizoctonia bataticola is world wide in distribution.
Biology and transmission
Sclerotia are black, smooth, hard and 0.1-1 mm diameter, and occur within roots, stems, leaves and fruits. Conidiomata are pycnidial, dark-brown, and either solitary or gregarious on leaves and stems; they are immersed, becoming erumpent, 100-200 µm diameter, opening by an apical ostiole; the conidiomatal wall is multicellular with heavily pigmented, thick-walled cells on the outermost side. Conidiophores are hyaline, short and obpyriform to cylindrical, 5-13 ´ 4-6 µm. Conidia are hyaline, ellipsoid to obovoid, 14-30 ´ 5-10 µm (Ahmed and Ravinder Reddy 1993). R. bataticola or M. phaseolina was detected in the seed coat, cotyledons and embryo of groundnut (Charabarty et al. 2005). It survives under different temperatures from -18°C to 20 °C temperatures (Singh et al. 2003).
Detection/indexing methods at ICRISAT
- Pre export field inspection and blotter test.
Treatment/control
- Seed treatment with a mixture of carbendazim and thiram (1:1) at 2 g a.i. kg-1 seed.
Procedures followed in case of positive test at ICRISAT
- If the seed colonization is <20% seed treatment with a mixture of carbendazim and thiram (1:1) at 2 g a.i. kg-1 is used, and seed samples having >20% colonization are rejected.
References and further reading
Ahmed KM, Ravinder Reddy Ch.1993. A Pictorial guide to the identification of seed borne fungi of sorghum, pearl millet, finger millet, chickpea, pigeonpea and groundnut. Information Bulletin No. 34. Patancheru, A.P. 502 324 India: International Crops Research Institute for the semi-Arid Tropics. 200 pp.
Chakrabarty SK, Girish AG, Anitha K, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Detection, seedborne nature, disease transmission and eradication of seedborne infection by Rhizoctonia bataticola (Taub.) Butler in groundnut, Indian Journal of Plant Protection 33: 85-89.
Chakrabarty SK, Anitha K, Girish AG, Sarath Babu B, Prasada Rao RDVJ, Varaprasad KS, Khetarpal RK, Thakur RP. 2005. Germplasm exchange and quarantine of ICRISAT mandate crops. Information Bulletin No. 69. Rajendranagar 500 030, Andhra Pradesh, India: National Bureau of Plant Genetic Resources; and Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi Arid Tropics. 80pp.
Sharma RC, Bhowmik TP. 1986. Estimation of yield losses in groundnut due to Macrophomina phaseolina (Tassi) Goid. Indian Journal of Plant Pathology 4:108-112.
Singh SD, Girish AG, Kameswar Rao N, Bramel PJ, Subhash Chandra. 2003. Survival of Rhizoctonia bataticola in groundnut seed under different storage conditions. Seed Science and technology Journal 31: 169-175.
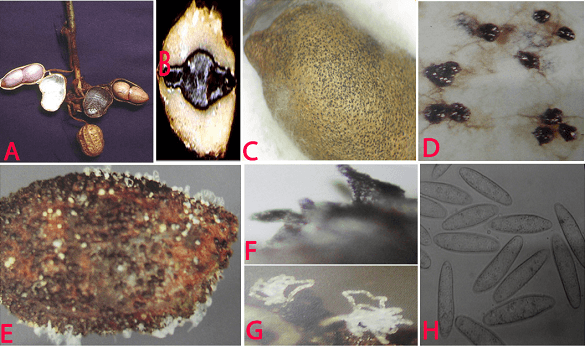 Charcoal rot (Rhizoctonia bataticola) of groundnut: (A)water soaked necrotic lesions on pods; (B)infected cotyledon; (C)infected seed with sclerotia; (D)sclerotia; (E)pycnidia and conidial ooze on seed; (F)pycnidia; (G)conidial ooze and (H)conidia of Macrophomina phaseolina (photos:ICRISAT) |